There are many names in the field of atomic model and atomic structure. The first name to understand the atomic structure of matter was J.J.Thomson who represented the Plum Pudding Model, that was based upon the concept that electrons in an atom are distributed on positive charge just like plums in a pudding. Ultimately making a sense like positive charge exists throughout the atom and negative electrons are unevenly distributed on it just like plums in the pudding. This concept of the atomic model is famous as plums in pudding model of atoms.
After that in 1899 new development came in the form of Ernest Rutherford Atomic Model. Ernst Rutherford was born at Nelson, New Zealand. He was educated at University of New Zealand and conducted research work in 1895 with J.J.Thomson at the Cavendish Laboratory . In 1907, he accepted longworthy Professorship at Manchester University in England where Chawdick was one of his students. He won Noble Prize in 1908 in the field of atomic physics.
Rutherford discovered alpha particles which are positively charged helium ions emitted from radioactive substances like uranium. During rutherford experiment it was discovered that alpha particles can create bright spots when they strike on a zinc sulphide coated screen.
As there is no concentrated mass in an atom, it was predicted that if a thin metallic foil is bombarded with positively charged alpha particles, then all such alpha particles would pass the foil without much deflection in their travelling path.
Rutherford’s Alpha Particles Scattering (gold foil) Experiment
Rutherford, Marsden and Hans Geiger conducted the experiment to verify the predictions and theories of Sir Rutherford. Their experiment was to bombard the alpha particles on a thin metallic foil. Under the guidance of Rutherford, Ernest Marsden and Hans Geiger conducted the required experiment.
A very thin gold film was placed in front of alpha ray gun. They also placed a zinc sulphide screen surrounding the gold film to observe the bright spots on it when alpha particles strike on it. As expected alpha particles were able to cross the film and striking on the zinc sulphide screen behind the film. Amazingly all the alpha particles did not cross the foil in the straight way as expected, instead small percentage of bombarded alpha particles changed their way of travel during crossing the gold foil.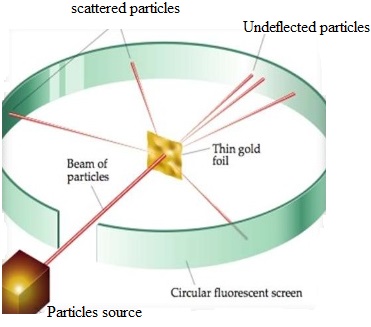
Rutherford concluded from the alpha particles scattering experiment that:
- Most of the space inside the atom is empty because most of the alpha particles passed through the gold foil without getting deflected.
- Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
- A very small fraction of alpha particles were deflected by 180 degree indicating that all the positive charges and mass of the gold atom were concentrated in a very small volume within the atom.
Ernest Rutherford Atomic Model Main Points
After Rutherford experiment as mentioned in previous section he gave a more realistic model of an atom. This model is called Rutherford’s Nuclear Atomic Model or Planetary Model of Atom. The main points of Rutherford’s nuclear model are described below:
- An atom consists of a positively charged nucleus, which is surrounded by electrons moving around it.
- Electrons and the nucleus are held together by coulombic force of attraction.
- The size of nucleus is very small as compared to the size of atom . Experimentally, it was found that , -14 -15 Radius of the nucleus of an atom=10 to 10 m Radius of an atom =10-10m Thus, the size of the nucleus is about ten thousandth part of the size of an atom.
- Almost the entire mass of an atom is concentrated in its nucleus.
- Atom , as a whole , is electrically neutral. So, number of protons inside the nucleus of an atom and the number of electrons surrounding the nucleus are equal.
Rutherford atomic model vs Thomson atomic model
Rutherford proposed a model in which electrons revolve around the nucleus in well – defined orbits . There is a positively charged centre in an atom called the Nucleus . He also proposed that the size of the nucleus is very small as compared to the size of the atom and nearly all the mass of an atom is centered in the nucleus.
Thomson proposed the model of an atom to be similar to a christmas pudding. The electrons are in a positively charged sphere like christmas pudding and the mass of the atom was supposed to be uniformly distributed .
The Rutherford’s Planetary Atomic Model
The radius of the nucleus is about 10-13 cm. The radius of circular path traveled by electrons around the nucleus is about 10-12 cm which is greater the diameter of an electron. The radius of the atom is about 10-8 cm. Thus the, like a planetary system, the atom is also of exceedingly open nature, due to which it can be penetrated by high-speed particles of various kinds. The Rutherford’s Planetary Atomic Model is shown in figure below:
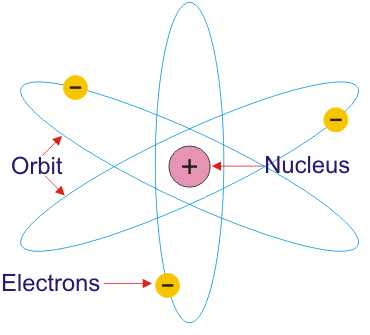
Rutherford’s Atomic model
Drawbacks of Rutherford’s Planetary Atomic Model
Rutherford’s atomic model does not obey the Maxwell theory of electrodynamics, according to it “a small charged particle moving around an oppositely charged centre continuously loses its energy”. If an electron does so, it should also continuously lose its energy and should set up spiral motion ultimately failing into the nucleus.
Rutherford could not explain how the moving electron could remain in its orbit, especially when it was charged particle and therefore it could accelerate due to its movement, finally moving closer to the nucleus and drop in to it. The atom would not be stable which in turn would mean that matter would not be composed of unstable atoms.

